Powder Coating Crosslinking Chemistry
In contrast to traditional liquid coating chemistries, only a small number of crosslinkers are employed to cure powder coatings. The base resin most commonly used for powder coatings is polyester (PER) that either carries hydroxyl or carboxyl functionalities that are reacted with four major crosslinking chemistries. Each has advantages and disadvantages which will be briefly discussed below.
Polyester/TGIC Crosslinking Systems
Triglycidylisocyanurate (TGIC) has been the predominate curing agent for polyester powder coatings since 1970. This hardener has three oxirane or glycidyl groups which react upon heating with the acid groups on the polyester. This type of chemistry can yield powder coatings with excellent weatherability and thus are ideal for outdoor use. Together with polyester-epoxy hybrids this chemistry has dominated the North American powder coating market.
The chemical reaction of the carboxylic functionality of a polyester resin with the oxirane functionality of TGIC is shown below. This type of reaction requires a bake temperature of at least 150°C or the coating will have poor mechanical properties and also might develop a haze commonly referred to as “blooming”. A typical bake profile is 10 - 15 minutes at 160°C (320°F).
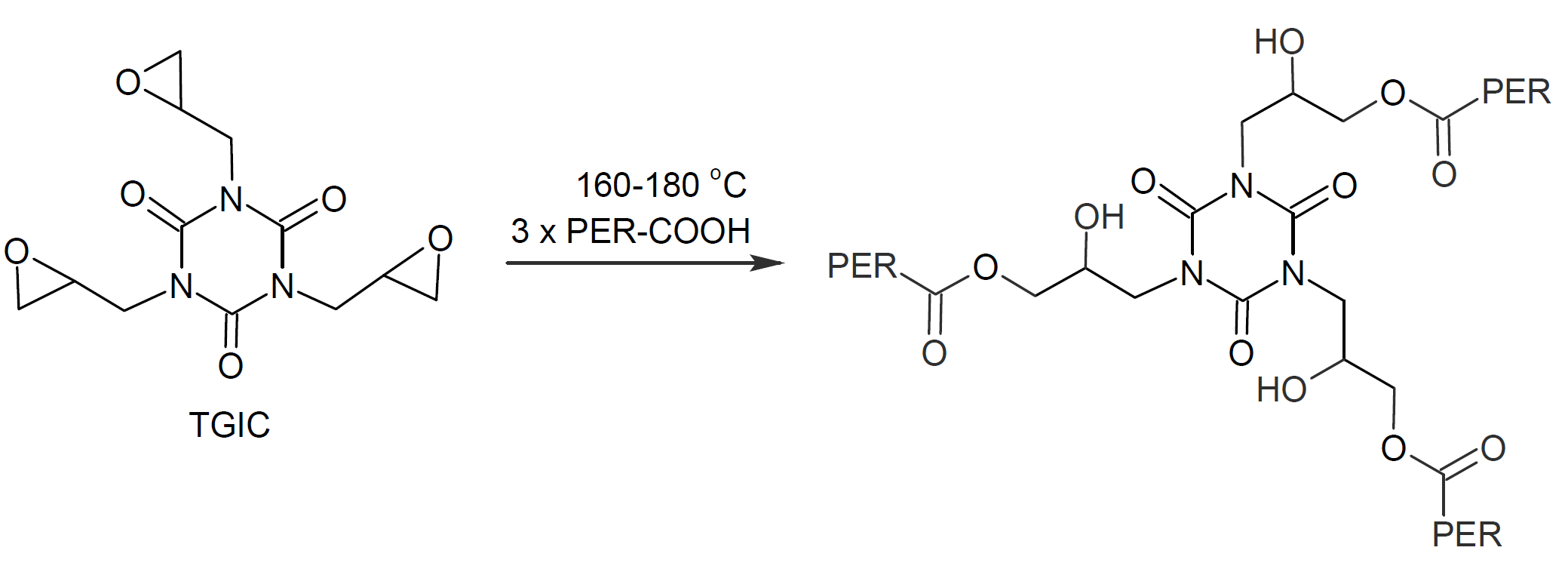
TGIC is considered a potential mutagen and therefore has to be labeled with skull-and-crossbones. To address its health and toxicity concerns, it has been replaced with other curing agents in many applications over the years, in particular with hydroxyalkylamide (HAA).
Hydroxyalkylamide (HAA) crosslinkers
Carboxyl functional polyesters can also be crosslinked with hydroxyl functional hardeners such as beta-hydroxyalkylamides. In fact, many European powder manufacturers have shifted from TGIC to this type of hardener to address health and toxicity concerns of TGIC. The most common hydroxyl hardener is N,N,N',N'-tetrakis-(2-hydroxyethyl)adipamide, often abbreviated to HAA. The esterification reaction between this hardener and carboxyl functionalities is shown belwo. It typically requires cure temperatures of at least 160°C.
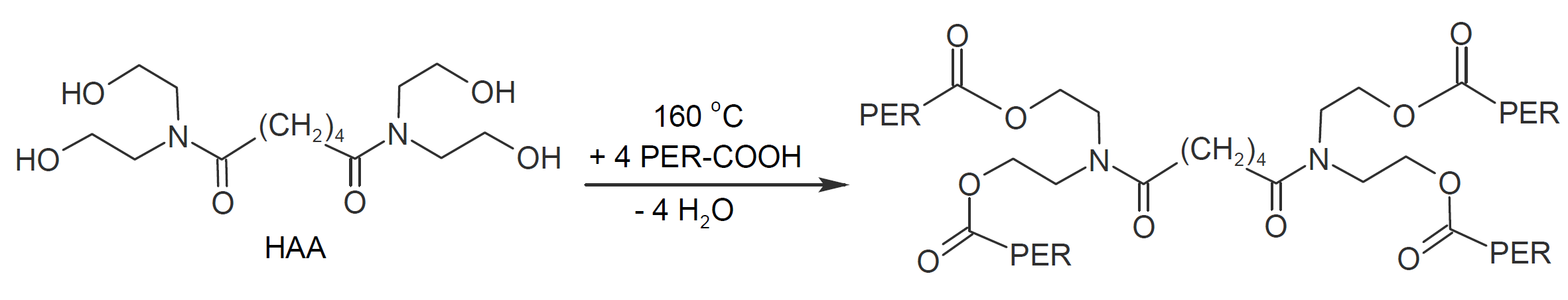
A potential drawback of this reaction is that hydroxyalkylamides liberate water during cure which can cause pin holing. To address this issue, special additives have been developed to allow for thick film cure without pin holing.
Blocked Polyisocyanate Crosslinkers
Polymeric blocked isocyanates are popular curing agents in the powder coating industry. They are frequently used to crosslink acrylic and polyester powder resins that carry hydroxyl functionalities. The most widely used polymeric isocyanate is isophorone diisocyanate (IPDI) and its higher functional adducts that are commonly blocked with ε-caprolactam which unblocks at temperatures around 180°C. To reduce the deblocking temperature and to accelerate cure, catalysts such as dibutyltin dilaurate (DBTDL) and zinc acetyl acetonate are typically added.
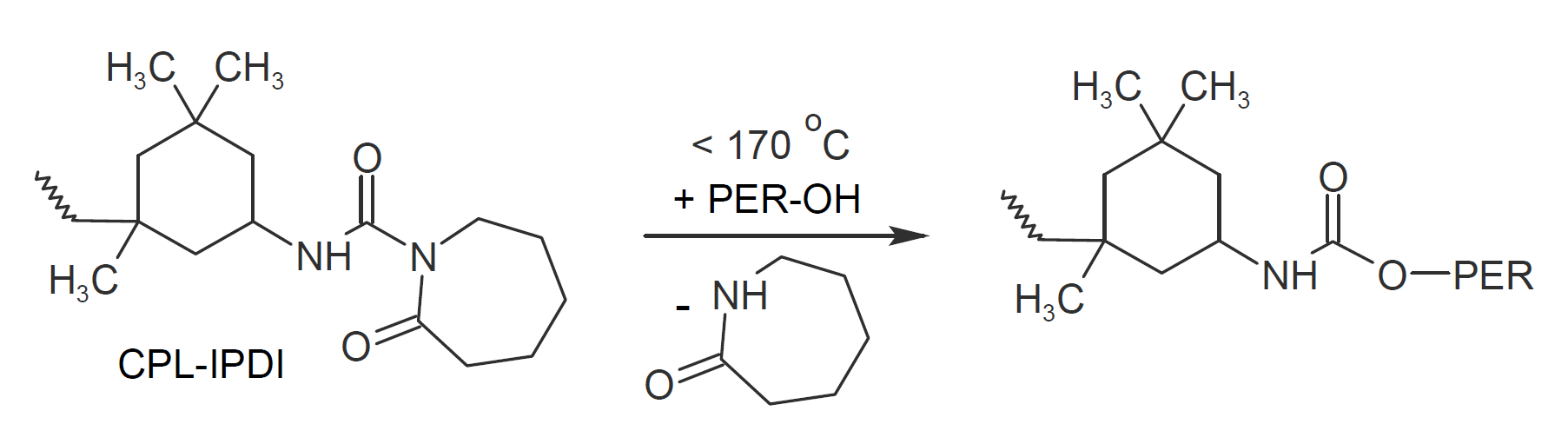
Two other commonly used polymeric blocked isocyanate curing agents are based on blocked methylene-bis-4-cyclohexyl diisocyanate and blocked toluene diisocyanate (TDI).
Hydroxyl funxtional powder resins can also be crosslinked with “self-blocked” isocyantes based on uretdione ring structure. These resins become reactive when the temperature exceeds the ring opening temperature of the uretdione. Since no blocking agent is released, they are considered non-emissive.
Glycoluril Crosslinkers (TMMGU)
Another common crosslinker for hydroxyl functional polyesters is tetramethoxymethyl glycouril (TMMGU), also known under the trade name Powderlink® 1174. This crosslinker is frequently used when a wrinkle or alligator finish is desired. Coating systems based on this crosslinking chemistry typically yield durable finishes with excellent mechanical properties and good weathering resistance. The condensation reaction between glycoluril and hydroxyl groups is shown below.
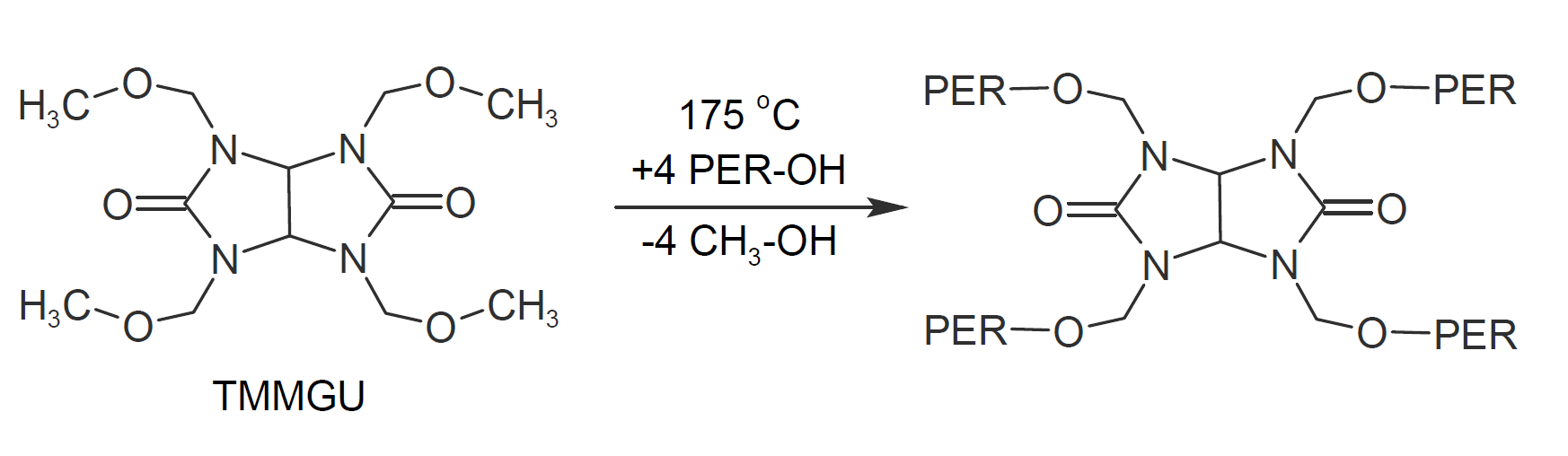
A major drawback of this reaction is the release of methanol which can produce pinholes during cure, particulalry in thicker films. To produce defect-free films with thicknesses in excess of 100 microns, special rheology control additives are typically required (US patent 5,256,713).